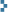Today’s long menu includes more radioactive pottery, more radioactive vacuum tubes, smoke detectors, a couple lesser-known radioactive elements, and a few interesting odds and ends. As always, if you have something radioactive and in need of a good home, I buy and trade all the time. Enjoy!

 Uranium-glazed artistic pottery is hard to come by, in contrast to the mass-produced (and mass-collected) Fiestaware and similar. Here are two examples of handmade ceramics. Especially interesting is a vase made in 2010 (left) that is representative of the work of crystalline-glaze artist William Melstrom, who has a studio in Austin, Texas (photo courtesy of Mr. Melstrom). Melstrom is one of very few contemporary artists who have gone to the lengths required nowadays to work with uranium. His adventuresome report on obtaining uranium compounds in France to formulate his glazes is a must-read. The fluorescent light yellow glaze on this vase clocks in at 2200 CPM on a 2″ pancake GM tube. At right is a hand-thrown and hand-glazed decorative bowl from an unknown artist containing a typical “uranium red” glaze. It registers 38,000 CPM on a 2″ pancake GM tube, making it among the hottest pieces of pottery in my collection.
Uranium-glazed artistic pottery is hard to come by, in contrast to the mass-produced (and mass-collected) Fiestaware and similar. Here are two examples of handmade ceramics. Especially interesting is a vase made in 2010 (left) that is representative of the work of crystalline-glaze artist William Melstrom, who has a studio in Austin, Texas (photo courtesy of Mr. Melstrom). Melstrom is one of very few contemporary artists who have gone to the lengths required nowadays to work with uranium. His adventuresome report on obtaining uranium compounds in France to formulate his glazes is a must-read. The fluorescent light yellow glaze on this vase clocks in at 2200 CPM on a 2″ pancake GM tube. At right is a hand-thrown and hand-glazed decorative bowl from an unknown artist containing a typical “uranium red” glaze. It registers 38,000 CPM on a 2″ pancake GM tube, making it among the hottest pieces of pottery in my collection.
.

 These raw ceramic underglazes containing uranium are a gift from William Melstrom, who made the vase pictured above. Before Melstrom owned them, they were in the possession of a radiation safety officer at the Texas Department of State Health Services, slated for official disposal as radioactive waste. Because so few artists use or even know about uranium glazes now, old bottles such as these sometimes present surprise disposal problems when studios are cleaned out. Both are products of Thompson Enamel and both read about 12,000 CPM on a 2″ pancake GM tube. At left is a “531 Burnt Orange” (when fired, of course), and at right is a “108 Forsythia.”
These raw ceramic underglazes containing uranium are a gift from William Melstrom, who made the vase pictured above. Before Melstrom owned them, they were in the possession of a radiation safety officer at the Texas Department of State Health Services, slated for official disposal as radioactive waste. Because so few artists use or even know about uranium glazes now, old bottles such as these sometimes present surprise disposal problems when studios are cleaned out. Both are products of Thompson Enamel and both read about 12,000 CPM on a 2″ pancake GM tube. At left is a “531 Burnt Orange” (when fired, of course), and at right is a “108 Forsythia.”
.


 This is a 6″ Corning uranium-glass optical filter I recently obtained on eBay. The uranium concentration is through the roof: it emits 11,000 CPM into a 2″ pancake GM tube, making it more than twice as hot as the hottest decorative vaseline glass items I own.
This is a 6″ Corning uranium-glass optical filter I recently obtained on eBay. The uranium concentration is through the roof: it emits 11,000 CPM into a 2″ pancake GM tube, making it more than twice as hot as the hottest decorative vaseline glass items I own.
Some other interesting properties of uranium glass are dramatically demonstrated with this example. In the second photo, ultraviolet light from a distant Sun-Kraft lamp (an electrodeless quartz-mercury discharge tube) excites the uranium glass, provoking the characteristic green fluorescence. Based absorption of the lamp’s harsh 254-nanometer UVC radiation, it’s easy to distinguish a quartz crucible (casting the central shadow) from the nearly-opaque borosilicate tube (left) and soda-lime glass vial (right).
Uranium glass is also apparently a fair scintillation medium. In the lower photo, a thin face of the Corning filter abuts the output window of a commercial x-ray machine, where exposure rates are on the order of 1000 roentgen / hour. The glass glows its characteristic green color as the x-ray beam expands across its surface.
.

 Lanthanum and lutetium are two of the lesser-known natural radioactive elements. Although there are other natural, primordial radioelements (e.g. V-50, Rb-87, Sm-147, Re-187, In-115), these two stand out (along with good old potassium) for their usefully high gamma activity. Both could be used as check sources or energy calibration sources for scintillation detectors. La-138 (0.09% abundance, T1/2 = 1.02E+11 y) decays by electron capture or beta emission, unleashing gamma rays in either branch. A ~50-g specimen of the metal (inset, left) racked up 7.2 counts / sec above background into a 2″ NaI:Tl detector. Lu-176 (2.6% abundance, T1/2 = 3.78E+10 y) undergoes beta decay with a high yield of several gamma energies, most notably at 202 and 307 keV. The peak at 509 keV in the spectrum is not a real gamma energy, but rather a “sum peak” caused by 202- and 307-keV gammas simultaneously entering the detector (this happens to be an “anomalous” sum peak, larger than would occur by random summation, precisely because the two radiations involved are frequently part of the same decay sequence). The 23-g chunk of lutetium in the right inset veritably boils a 2″ NaI:Tl detector with more than 120 counts / sec above background.
Lanthanum and lutetium are two of the lesser-known natural radioactive elements. Although there are other natural, primordial radioelements (e.g. V-50, Rb-87, Sm-147, Re-187, In-115), these two stand out (along with good old potassium) for their usefully high gamma activity. Both could be used as check sources or energy calibration sources for scintillation detectors. La-138 (0.09% abundance, T1/2 = 1.02E+11 y) decays by electron capture or beta emission, unleashing gamma rays in either branch. A ~50-g specimen of the metal (inset, left) racked up 7.2 counts / sec above background into a 2″ NaI:Tl detector. Lu-176 (2.6% abundance, T1/2 = 3.78E+10 y) undergoes beta decay with a high yield of several gamma energies, most notably at 202 and 307 keV. The peak at 509 keV in the spectrum is not a real gamma energy, but rather a “sum peak” caused by 202- and 307-keV gammas simultaneously entering the detector (this happens to be an “anomalous” sum peak, larger than would occur by random summation, precisely because the two radiations involved are frequently part of the same decay sequence). The 23-g chunk of lutetium in the right inset veritably boils a 2″ NaI:Tl detector with more than 120 counts / sec above background.
.

 More radioactive vacuum tubes. At right are three similar radar TR switches and their packaging (left to right: Bomac JAN-CBNQ-5883 from 1961 originally containing 0.3 µCi of Co-60; a Westinghouse 1B37 from 1952 containing several µCi or Ra-226; a GE 1B35 containing a small amount of Co-60. At left, a spark gap (in hand) originally with 5 µCi of Cs-137 and a dual TR switch originally containing less than 0.7 µCi of Co-60.
More radioactive vacuum tubes. At right are three similar radar TR switches and their packaging (left to right: Bomac JAN-CBNQ-5883 from 1961 originally containing 0.3 µCi of Co-60; a Westinghouse 1B37 from 1952 containing several µCi or Ra-226; a GE 1B35 containing a small amount of Co-60. At left, a spark gap (in hand) originally with 5 µCi of Cs-137 and a dual TR switch originally containing less than 0.7 µCi of Co-60.
.


 Ionization smoke detectors contain an alpha emitter, typically Am-241. The left-most pic shows industrial smoke detectors from ca. 1960, each containing a total of 80 microcuries of Am-241. These detectors measured the current imbalance between an exposed “sense chamber” and a sealed “reference chamber,” both of which contained alpha sources. In front of the detectors are examples of their sense-chamber sources, which hold the greater amount of activity (~60 microcuries). Left is a Pyrotronics F5-B4 with its annular source holder bearing six thin sealed sources; at right is an F3/5A and its pedestal source, containing a single foil covered by a screw-adjustable bonnet. More modern detectors are shown in the upper-right image: At left is a Simplex 2098-9508 with 4.5 µCi of Am-241, manufactured in 1980, and at right a run-of-the-mill modern detector with the typical 1-µCi source. The lower right photo shows a Ra-226 foil source from a batch of smoke detectors, make unknown, that was intercepted on its way into a Pennsylvania junkyard. Approximate activity is 1 microcurie.
Ionization smoke detectors contain an alpha emitter, typically Am-241. The left-most pic shows industrial smoke detectors from ca. 1960, each containing a total of 80 microcuries of Am-241. These detectors measured the current imbalance between an exposed “sense chamber” and a sealed “reference chamber,” both of which contained alpha sources. In front of the detectors are examples of their sense-chamber sources, which hold the greater amount of activity (~60 microcuries). Left is a Pyrotronics F5-B4 with its annular source holder bearing six thin sealed sources; at right is an F3/5A and its pedestal source, containing a single foil covered by a screw-adjustable bonnet. More modern detectors are shown in the upper-right image: At left is a Simplex 2098-9508 with 4.5 µCi of Am-241, manufactured in 1980, and at right a run-of-the-mill modern detector with the typical 1-µCi source. The lower right photo shows a Ra-226 foil source from a batch of smoke detectors, make unknown, that was intercepted on its way into a Pennsylvania junkyard. Approximate activity is 1 microcurie.
.


 Tritium glow-in-the-dark devices include emergency exit signage and the button at right. Self-luminous exit signs are undoubtedly the most radioactive items in peoples’ everyday experience, but few probably realize it. They can contain up to 20 curies of H-3 (tritium) gas in the glowing phosphor-lined tubes, as does the example shown here. They are regulated under a General License by the Nuclear Regulatory Commission (see yellow sticker in right image). Though initially costly, these self-powered signs easily deliver value over the life of a building by eliminating the need to conduct tests and change light bulbs. Numerous outlets sell them on the Internet; they can also frequently be found at bargain prices on eBay (when the NRC isn’t looking). The lower pic shows an old luminous button that originally contained 0.1 Ci of tritium. This item replaced more hazardous predecessors containing radium. Common consumer goods containing tritium today include “Traser” keychain lights (technically illegal in the USA as a “frivolous use” of radioactive material) and Trijicon gun sights.
Tritium glow-in-the-dark devices include emergency exit signage and the button at right. Self-luminous exit signs are undoubtedly the most radioactive items in peoples’ everyday experience, but few probably realize it. They can contain up to 20 curies of H-3 (tritium) gas in the glowing phosphor-lined tubes, as does the example shown here. They are regulated under a General License by the Nuclear Regulatory Commission (see yellow sticker in right image). Though initially costly, these self-powered signs easily deliver value over the life of a building by eliminating the need to conduct tests and change light bulbs. Numerous outlets sell them on the Internet; they can also frequently be found at bargain prices on eBay (when the NRC isn’t looking). The lower pic shows an old luminous button that originally contained 0.1 Ci of tritium. This item replaced more hazardous predecessors containing radium. Common consumer goods containing tritium today include “Traser” keychain lights (technically illegal in the USA as a “frivolous use” of radioactive material) and Trijicon gun sights.
.
 Kodak 8-mm film projector (left) and camera (right) with radioactive thorium lenses. High refractive index and low dispersion justified the use of thoria in optical glass formulations. The film projector’s 22-mm, f/1.0 Projection Ektar lens clocks in at 1200 CPM on contact with a 2″ pancake GM tube, while the camera’s lens only reads about 250 CPM.
Kodak 8-mm film projector (left) and camera (right) with radioactive thorium lenses. High refractive index and low dispersion justified the use of thoria in optical glass formulations. The film projector’s 22-mm, f/1.0 Projection Ektar lens clocks in at 1200 CPM on contact with a 2″ pancake GM tube, while the camera’s lens only reads about 250 CPM.
.
 Radium postcard, ca. 1930, from Luther Gable quack outfit. Ah, the good old days when you could just send loose radioactive contamination through the freaking mail! This postcard bearing a dollop of glow-in-the-dark radium paint (11,000 CPM on a 2″ pancake GM tube) promoted Dr. Luther Gable, the man responsible for the notorious Gable Ionic Charger. A number of these cards were found in a collection of magician’s tricks.
Radium postcard, ca. 1930, from Luther Gable quack outfit. Ah, the good old days when you could just send loose radioactive contamination through the freaking mail! This postcard bearing a dollop of glow-in-the-dark radium paint (11,000 CPM on a 2″ pancake GM tube) promoted Dr. Luther Gable, the man responsible for the notorious Gable Ionic Charger. A number of these cards were found in a collection of magician’s tricks.
.
 The “Becquerel Chemicals” educational kit manufactured by Damon contains six small plastic boxes labeled A through F. The contents of three are yellow powders, the contents of the other three are white crystals. Students were intended to exploit physical and chemical properties—including radioactivity—to identify these unknowns from a list consisting of uranyl sulfate, sodium sulfate, uranyl nitrate, sodium nitrate, thorium nitrate, and sulfur.
The “Becquerel Chemicals” educational kit manufactured by Damon contains six small plastic boxes labeled A through F. The contents of three are yellow powders, the contents of the other three are white crystals. Students were intended to exploit physical and chemical properties—including radioactivity—to identify these unknowns from a list consisting of uranyl sulfate, sodium sulfate, uranyl nitrate, sodium nitrate, thorium nitrate, and sulfur.